Reaktions-Schema
Castner Reaktor
Zur Herstellung von Natrium
Kathode
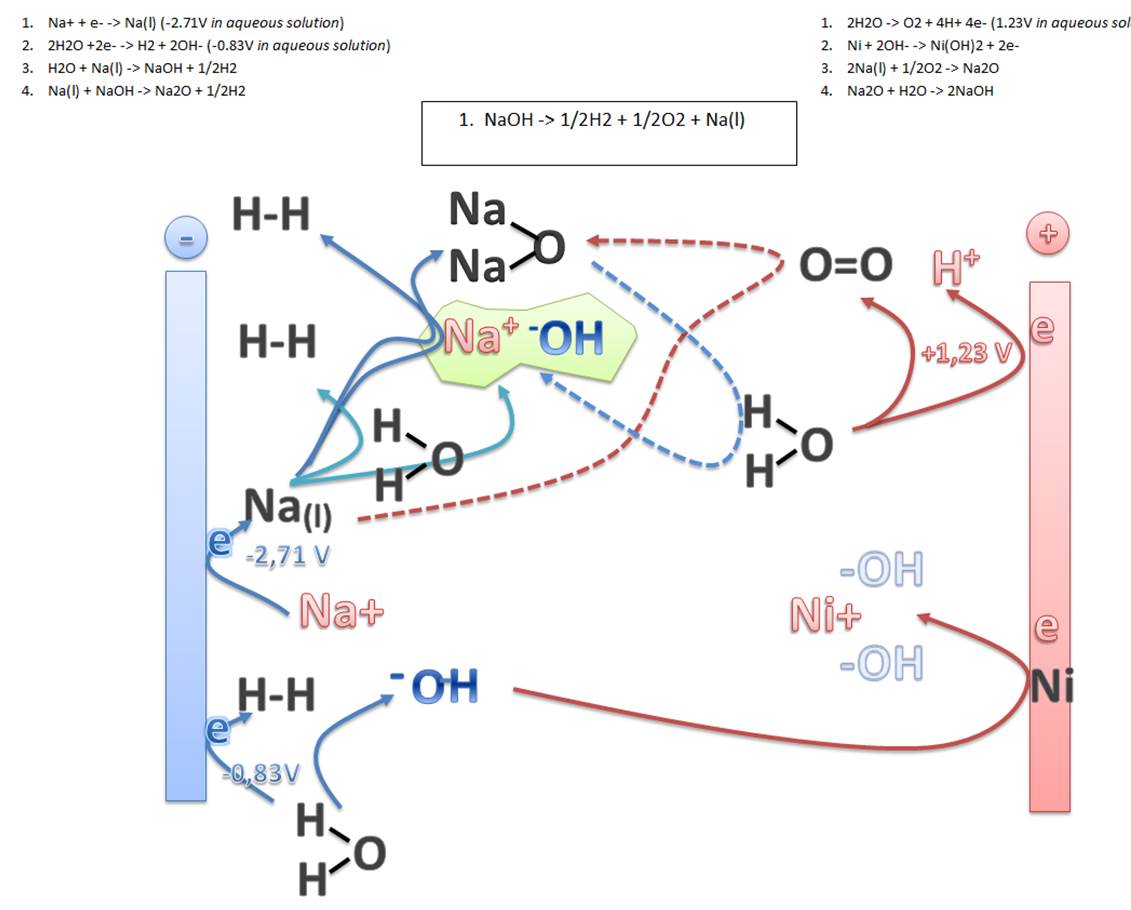
Na+ + e- -> Na(l) (-2.71V in aqueous solution)
2H2O +2e- -> H2 + 2OH- (-0.83V in aqueous solution)
H2O + Na(l) -> NaOH + 1/2H2
Na(l) + NaOH -> Na2O + 1/2H2
Anode
2H2O -> O2 + 4H+ 4e- (1.23V in aqueous solution)
Ni + 2OH- -> Ni(OH)2 + 2e-
2Na(l) + 1/2O2 -> Na2O
Na2O + H2O -> 2NaOH
Castner Reaktor Chemie Schema
Quelle
http://www.sciencemadness.org/talk/viewthread.php?tid=9797
The following are the major reactions occuring at the cathode
Na+ + e- -> Na(l) (-2.71V in aqueous solution)
2H2O +2e- -> H2 + 2OH- (-0.83V in aqueous solution)
H2O + Na(l) -> NaOH + 1/2H2
Na(l) + NaOH -> Na2O + 1/2H2
The following are the major reactions occuring at the anode
2H2O -> O2 + 4H+ 4e- (1.23V in aqueous solution)
Ni + 2OH- -> Ni(OH)2 + 2e-
2Na(l) + 1/2O2 -> Na2O
Na2O + H2O -> 2NaOH
The overall useful reaction is
NaOH -> 1/2H2 + 1/2O2 + Na(l)
|
|