Recherche Methan Herstellung
Untersuchung zu patentierten Methoden zur Hestellung von Methan aus CO2 und alternativen
|
Im Detail leider uneffektiv
Process for generation of synthetic fuel from carbonaceus substances
US 8536234 B2
Quelle :USPTO
VonAmerol Enterprises, Llc
Jahr : 2010
ZUSAMMENFASSUNG
A method and apparatus for the
generation of synthetic motor fuels
and additives to oil fuels, C1-C4alcohols, hydrogen,methane, synthesized gas (H2+CO2)
by hydrothermal treatment of carbonaceous compounds
by providing a two-stage carbon gasification process operated underthe supercritical conditions of H2O and CO2, including a first stage gasification reactor having a reaction zone for the conversion of carbonaceous compounds and a second stage reactor for the conversion of the products of the first stage reactor;
feeding aaqueous suspension of carbonaceous compoundin an amount of at least 30% by weight
and an alkali metal oralkaline-earth metal catalystor reactive OH-species
from an electrolyzer
through said
first stage gasification reactor as a supercritical fluid at a volume velocity of0.01-0.05 g of carbon per 1 cm3per hour, at a carbon/catalyst mole ratio of between about 70/1 and 90/1, at atemperatureof 390-450° C., and under a pressure of about 225 to 500 bars; feeding the reaction products from the first stage reactor to the second stage reactor over a copper-zinc catalyst at thetemperatureof 200-280° C. and under a pressure of at least about 100 bars so that any gases generated in the first stage are converted into C1-C4alcohols with the weight ratio of C1-C2to C3-C4between about 0-35% to 100-65%.
|

|
Auch aus China kommen Top Produkte
UNIV SICHUAN
Jahr 2012
Catalyst for carbon dioxide methanation and preparation method thereof
Catalyst for carbon dioxide methanation and preparation method thereof
CN 102600854 A
ZUSAMMENFASSUNG
The invention discloses a catalyst for carbon dioxide methanation and a preparation method of the catalyst, belonging to the technical field of carbon dioxide methanation. The catalyst for carbon dioxide methanation is composed of a composite carrier and an active ingredient at a ratio of 84-90wt%:10-16wt%, wherein the composite carrier is composed of gamma-Al2O3 and water soluble metal oxide at a mass ratio of 77-86:2-10; and the active ingredient is Ni which exists in the catalyst in a form of NiO. The catalyst is high in activity, low in cost and better in stability, and can be used for carbon dioxide methanation reaction under normal pressure condition.
1 Carbon dioxide methanation catalyst, characterized in that the carrier and the active component from the composite composition, composite carrier: the active component = 84-90wt%: 10-16wt%; wherein said composite carrier with water, the Y-Al2O3 metal oxides, Y-Al2O3 with a water soluble metal oxide weight ratio of 77-86: 2-10; said active component is Ni, and NiO present in the catalyst.
(2) as claimed in claim I wherein the carbon dioxide methanation catalyst, wherein said water-soluble metal oxide is Ce02, ZrO2, or at least one of La2O3.
3 according to Claim I or 2, wherein the carbon dioxide methanation catalyst, wherein the catalyst mass ratio of the components: compound Carrier: active ingredient = 84-88: 12-16; composite vector Y -Al2O3 with a water soluble metal oxide weight ratio of 78-86: 2-6.
|

|
Method for producing fuel from captured carbon dioxide
CA 2579133 A1
2005
Aytec Avnim Ltd
ZUSAMMENFASSUNG
The invention provides a method for producing combustible fuels from a gaseous mixture containing carbon dioxide, which comprises: (i) capturingCO2from said gaseous mixture by means of K2CO3, thus forming KHCO3; (ii) releasing theCO2from said KHCO3; and (iii) subsequently producing fuel from the releasedCO2by reaction with hydrogen.
1. A method for producing combustible fuels from a gaseous mixture containing carbon dioxide, which comprises:
(i) capturing CO2 from said gaseous mixture by means of K2CO3, thus forming KHCO3;
(ii) releasing the CO2 from said KHCO3 ; and (iii) subsequently producing fuel from the released CO2 by reaction with hydrogen.
2. The method of claim 1, wherein said gaseous mixture is air.
3. The method of claim 2, wherein the capture of CO2 is performed by bubbling air in water through an aqueous solution of K2CO3.
…dazwischen (Quatsch)
10. The method of claim 9, wherein said electrochemical reaction corresponds to a reverse operation of adirect methanol fuel cell(DMFC) and the fuel produced is methanol.
11. The method of claim 6, wherein said electrochemical reaction corresponds to a reverse operation of a molten carbonate fuel cell (MCFC) and the fuel produced is a hydrocarbon such asmethane.
|
|
Plasma formed in a fluid
US 20060060464 A1
UnterCA2483753A1
VonChang Chak M T
Jahr 2003
ZUSAMMENFASSUNG
A method and apparatus for generating plasma in a fluid. The fluid (3) is placed in a bath (2) having a pair of spaced electrodes (4, 6) forming a cathode and an anode. A stream of bubbles is introduced or generated within the fluid adjacent to the cathode. A potential difference is applied across the cathode and anode such that a glow discharge is formed in the bubble region and a plasma of ionized gas molecules is formed within the bubbles. The plasma may then be used in electrolysis, gas production, effluent treatment or sterilization, mineral extraction, production of nanoparticles or material enhancement. The method can be carried out at atmospheric pressure and roomtemperature. The electrodes may carry means to trap the bubbles in close proximity.Partitions may be present between the electrodes.
|

|
Electrochemical reactor forco2conversion, utilization and associated carbonate electrocatalyst
WO 2012061728 A2
2010
VonUniversity Of Connecticut
US20120193222
ZUSAMMENFASSUNG
Electrochemical reactors are provided that operate on the carbonate cycle at extremely low temperatures (e.g., less than 50°C), thereby allowing operation in as many as three (3) modes, namely as: (i) a roomtemperaturecarbonate fuel cell; (ii) an electrochemically assisted CO2membrane separator; and (iii) a CO2conversion device. Electrocataiysts are also provided that have the ability to selectively form carbonate anions over hydroxide anions under fully humidified conditions.Exemplary electrocataiysts according to the present disclosure include pyrochlores
|
|
Fischer-tropsch catalysts containing iron or cobalt selective towards higher hydrocarbons
US 20120245236 A1
2011
EP2691174A1
ZUSAMMENFASSUNG
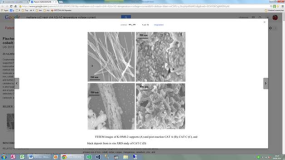
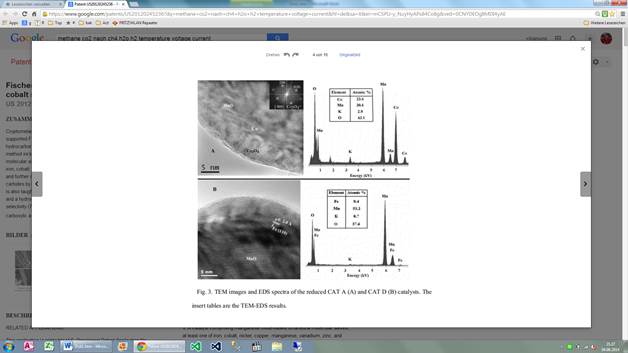
Cryptomelane-type manganese oxide octahedral molecular sieves (OMS-2) supported Fe and Co catalysts are utilized in a method for producing hydrocarbons by a Fischer-Tropsch mechanism. The hydrocarbon producing method includes providing a catalyst of a manganese oxide-based octahedral molecular sieve nanofibers with an active catalyst component of at least one of iron, cobalt, nickel, copper, manganese, vanadium, zinc, and mixtures thereof, and further containing an alkali metal. The formation of iron carbides and cobalt carbides by exposing the catalyst to conditions sufficient to form those carbides is also taught. After the catalyst has been appropriately treated, a carbon source and a hydrogen source are provided and contacted with the catalyst to thereby form a hydrocarbon containing product. The catalyst have high catalytic activity and selectivity (75%) for C2+ hydrocarbons in both CO hydrogenation andCO2hydrogenation. Highly selective syntheses of high value jet fuel, C2-C6 alkenes, C2-C6 carboxylic acids;α-hydroxylic acids and their derivatives have been realized by tuning the oxidation ability of OMS-2 supports and by doping with Cu2+ions.
|
|
|
|